A comment from Professor Sheila Bird on data from the UK vaccine roll-out in case useful.
Prof Sheila Bird, Formerly Programme Leader, MRC Biostatistics Unit, University of Cambridge, said:
“I am calling for more detailed breakdown and analysis of UK vaccination data. The data provided should include: vaccine type received, gender, age-group, region, weeks of 1st dose, whether 2nd dose was received and the dose interval.
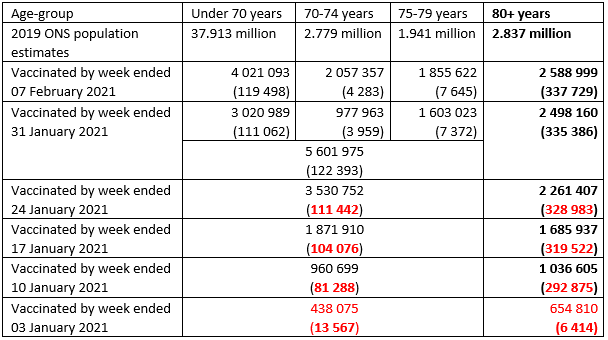
“We need to be able to analysis the dosing-strategy the UK took. 650,000 80+ year olds in England received their 1st Pfizer/BioNTech dose before 4 January 2021 and about half of them received their 2nd dose by 24th January 2021 (see Table). This ‘Serendipity Cohort’ of elderly people with 2 doses needs sophisticated analysis to be able to compare the different dosing strategies, since half received their 2nd dose within 4 weeks of the 1st, the remainder within 12 weeks. The analysis also needs to factor in the dramatically changing SARS-CoV-2 incidence and falling hospitalizations during January and February 2021. Where is this analysis to be found?
“A similar analysis is also required for the ‘Care Cohort’, roughly 440,000 younger persons, mainly health and social care workers, whose 1st dose of Pfizer/BioNTech vaccine was administered prior to 4th January 2021, one-quarter of whom received their 2nd dose of Pfizer/BioNTech vaccine within 4 weeks of the first.
“We are now more than 12 weeks after 4 January 2021 so that those vaccinated with the first dose before that date will have by now received their 2nd dose of Pfizer/BioNTech vaccine. By 4 April, in theory, all would be fully protected. However, both cohorts now need careful, ongoing analysis to infer the impact of the UK’s decision, taken in the public interest, to delay the administration of the 2nd dose from 3 weeks (as in Pfizer/BioNTech randomized controlled trial) to 12 weeks.
“Analysing the effect of the different dose-intervals for the Pfizer vaccine is now all the more critical because, in mid-March, the National Institute for Health Research quietly suspended its research-call (after receipt of applications) for randomized comparison of inter-dose-interval for COVID-vaccines on short and longer-term outcomes. Applicants’ study-design had to be capable of discerning a 20-percentage point difference in the incidence of SARS-CoV-2 cases and/or hospitalizations.
“As well as data and analysis on the dosing interval I would also like to see data separated by the different vaccine types. Vaccine type matters – Scotland’s official statistics recognise this, yet, NHS England’s website on vaccinations from 8 December 2020 still fails to differentiate by vaccine-type, see https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-vaccinations/.
“Worse, the UK government’s website (https://coronavirus.data.gov.uk) appears to re-write history by omitting all daily vaccine counts prior to 11 January 2021! But there are over one million English citizens who received their 1st dose of PfizerBioNTech vaccine before 4 January 2021, of whom 40% had received their 2nd dose within 4-weeks (mostly before 11 January 2021) who are not explicit on the gov.uk record.
“Another important context in which vaccine-type matters is projections. Modelling papers for SAGE and SPI-M-O released on 31 March 2021 included three impressive sets of projections for how SARS-CoV-2 cases, hospitalizations and deaths could evolve when England progresses beyond limited openings-up on 12 April to further steps on 17 May 2021 and in late June. The models make different assumptions by vaccine-type about effectiveness after 1st and 2nd dose. Assumptions about vaccine effectiveness for reducing symptomatic SARS-CoV-2 cases differ by 20 percentage points between vaccine types and the teams also differ in whether Oxford/AstraZeneca vaccine will account for 70% or 80% of future 1st doses. These models warn us to expect that the majority of hospitalizations will be those who have been vaccinated but who have not been adequately protected by their vaccination: these individuals, in the most vulnerable groups, are predicted to outnumber those who are unvaccinated. For the over 60s, the die is now largely cast in terms of vaccine-type. Not so for younger citizens.
“Modellers need to know vaccine-type to apply different assumptions about vaccine effectiveness by vaccine-type. In addition to vaccine-type, the data provided should include: gender, age-group, region, weeks of 1st dose, whether 2nd dose was received, and dose-interval. Strangely, dose-interval seems not to be provided because dose-interval was assumed at 11 weeks by one team. If data on dose-interval are not provided, they cannot be analysed.
“This lack of data provision on dose-interval for the PfizerBioNTech vaccine makes it harder to create accurate models – the expertise of the modellers is not the limiting factor!
Table 1: England’s roll-out of COVID-19 vaccines by week – cumulative 1st does (2nd doses). In red: doses which are necessarily Pfizer/BioNTech
All our previous output on this subject can be seen at this weblink:
www.sciencemediacentre.org/tag/covid-19
Declared interests
Prof Sheila Bird: “SMB served on UK’s Medicines Commission (1991-1995); supervised the doctoral thesis on pharmacovigilance in Uganda of Dr Ronald Kiguba; and gave Edinburgh University’s David Finney Centenary Lecture in 2017.”